
HL Paper 2
Chlorine undergoes many reactions.
of manganese(IV) oxide was added to of .
Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
is a common chlorofluorocarbon, .
State the full electron configuration of the chlorine atom.
State, giving a reason, whether the chlorine atom or the chloride ion has a larger radius.
Outline why the chlorine atom has a smaller atomic radius than the sulfur atom.
The mass spectrum of chlorine is shown.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Outline the reason for the two peaks at and .
Explain the presence and relative abundance of the peak at .
Calculate the amount, in , of manganese(IV) oxide added.
Determine the limiting reactant, showing your calculations.
Determine the excess amount, in , of the other reactant.
Calculate the volume of chlorine, in , produced if the reaction is conducted at standard temperature and pressure (STP). Use section 2 of the data booklet.
State the oxidation state of manganese in and .
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
State the formula of the conjugate base of hypochlorous acid.
Calculate the concentration of in a solution with a .
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
Predict, giving a reason, whether ethane or chloroethane is more reactive.
Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, , using curly arrows to represent the movement of electron pairs.
Ethoxyethane (diethyl ether) can be used as a solvent for this conversion.
Draw the structural formula of ethoxyethane
Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet.
Calculate the percentage by mass of chlorine in .
Comment on how international cooperation has contributed to the lowering of emissions responsible for ozone depletion.
s produce chlorine radicals. Write two successive propagation steps to show how chlorine radicals catalyse the depletion of ozone.
Markscheme
✔
Do not accept condensed electron configuration.
AND more «electron–electron» repulsion ✔
Accept AND has an extra electron.
has a greater nuclear charge/number of protons/ «causing a stronger pull on the outer electrons» ✔
same number of shells
OR
same «outer» energy level
OR
similar shielding ✔
«two major» isotopes «of atomic mass and » ✔
«diatomic» molecule composed of «two» chlorine-37 atoms ✔
chlorine-37 is the least abundant «isotope»
OR
low probability of two «isotopes» occurring in a molecule ✔
✔
✔
AND is the limiting reactant ✔
Accept other valid methods of determining the limiting reactant in M2.
✔
✔
Accept methods employing .
✔
✔
oxidizing agent AND oxidation state of changes from to /decreases ✔
partially dissociates/ionizes «in water» ✔
✔
✔
«free radical» substitution/ ✔
Do not accept electrophilic or nucleophilic substitution.
chloroethane AND C–Cl bond is weaker/ than C–H bond/
OR
chloroethane AND contains a polar bond ✔
Accept “chloroethane AND polar”.
curly arrow going from lone pair/negative charge on in −OH to ✔
curly arrow showing leaving ✔
representation of transition state showing negative charge, square brackets and partial bonds ✔
Accept with or without the lone pair.
Do not accept curly arrows originating on in .
Accept curly arrows in the transition state.
Do not penalize if and are not at 180°.
Do not award M3 if bond is represented.
/ ✔
Accept .
2 «signals» ✔
0.9−1.0 AND triplet ✔
3.3−3.7 AND quartet ✔
Accept any values in the ranges.
Award [1] for two correct chemical shifts or two correct splitting patterns.
✔
✔
Award [2] for correct final answer.
Any of:
research «collaboration» for alternative technologies «to replace s»
OR
technologies «developed»/data could be shared
OR
political pressure/Montreal Protocol/governments passing legislations ✔
Do not accept just “collaboration”.
Do not accept any reference to as greenhouse gas or product of fossil fuel combustion.
Accept reference to specific measures, such as agreement on banning use/manufacture of s.
✔
OR
✔
Penalize missing/incorrect radical dot (∙) once only.
Examiners report
Well answered question with 90% of candidates correctly identifying the complete electron configuration for chlorine.
Most candidates could correctly explain the relative sizes of chlorine atom and chloride ion.
Fairly well answered though some candidates missed M2 for not recognizing the same number of shells affected.
More than 80% could identify that the two peaks in the MS of chlorine are due to different isotopes.
Not well answered. Some candidates were able to identify m/z 74 being due to the m/z of two Cl-37 atoms, however fewer candidates were able to explain the relative abundance of the isotope.
Stoichiometric calculations were generally well done and over 90% could calculate mol from a given mass.
90% of candidates earned full marks on this 2-mark question involving finding a limiting reactant.
Surprisingly, quite a number of candidates struggled with the quantity of excess reactant despite correctly identifying limiting reactant previously.
Most candidates could find the volume of gas produced in a reaction under standard conditions.
More than 90% could identify the oxidation number of manganese in both MnO2 and MnCl2.
Most candidates stated that MnO2 is an oxidizing agent in the reaction but many did not get the mark because there was no reference to oxidation states.
Another well answered 1-mark question where candidates correctly identified a weak acid as an acid which partially dissociates in water.
Roughly ⅓ of the candidates failed to identify the conjugate base, perhaps distracted by the fact it was not contained in the equation given.
Vast majority of candidates could calculate the concentration of H+ (aq) in a HClO (aq) solution with a pH =3.61.
Many identified the reaction of chlorine with ethane as free-radical substitution, or just substitution, with some erroneously stating nucleophilic or electrophilic substitution.
The underlying reasons for the relative reactivity of ethane and chloroethane were not very well known with a few giving erroneous reasons and some stating ethane more reactive.
Few earned full marks for the curly arrow mechanism of the reaction between sodium hydroxide and chloroethane. Mistakes being careless curly arrow drawing, inappropriate –OH notation, curly arrows from the hydrogen or from the carbon to the C–Cl bond, or a method that missed the transition state.
Approximately 60% could draw ethoxyethane however many demonstrated little knowledge of structure of an ether molecule.
A poorly answered question with some getting full marks on this 1HNMR spectrum of ethoxyethane question. Very few could identify all 3 of number of signals, chemical shift, and splitting pattern.
Another good example of candidates being well rehearsed in calculations with 90% earning 2/2 on this question of calculation percentage by mass composition.
Somewhat disappointing answers on this question about how international cooperation has contributed to the lowering of CFC emissions. Many gave vague answers and some referred to carbon emissions and global warming.
Few could construct the propagation equations showing how CFCs affect ozone, and many lost marks by failing to identify ClO· as a radical.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
Describe how the relative atomic mass of a sample of calcium could be determined from its mass spectrum.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Sketch a graph of the first six ionization energies of calcium.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Describe how sigma (σ) and pi () bonds are formed.
Deduce the number of σ and bonds in a molecule of ethyne.
Markscheme
electrostatic attraction AND oppositely charged ions
[1 mark]
multiply relative intensity by «m/z» value of isotope
OR
find the frequency of each isotope
sum of the values of products/multiplication «from each isotope»
OR
find/calculate the weighted average
Award [1 max] for stating “m/z values of isotopes AND relative abundance/intensity” but not stating these need to be multiplied.
[2 marks]
«promoted» electrons fall back to lower energy level
energy difference between levels is different
Accept “Na and Ca have different nuclear charge” for M2.
[2 marks]
Any two of:
stronger metallic bonding
smaller ionic/atomic radius
two electrons per atom are delocalized
OR
greater ionic charge
greater atomic mass
Do not accept just “heavier” or “more massive” without reference to atomic mass.
[2 marks]
delocalized/mobile electrons «free to move»
[1 mark]
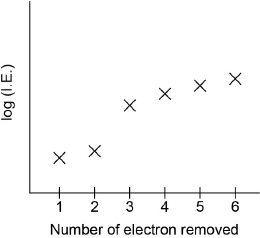
general increase
only one discontinuity between “IE2” and “IE3”
[2 marks]
pH > 7
Accept any specific pH value or range of values above 7 and below 14.
[1 mark]
sigma (σ):
overlap «of atomic orbitals» along the axial/internuclear axis
OR
head-on/end-to-end overlap «of atomic orbitals»
pi ():
overlap «of p-orbitals» above and below the internuclear axis
OR
sideways overlap «of p-orbitals»
Award marks for suitable diagrams.
[2 marks]
sigma (σ): 3
AND
pi (): 2
[1 mark]
Examiners report
Bromine can form the bromate(V) ion, BrO3−.
State the electron configuration of a bromine atom.
Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided. Use boxes to represent orbitals and arrows to represent electrons.
Draw two Lewis (electron dot) structures for BrO3−.
Determine the preferred Lewis structure based on the formal charge on the bromine atom, giving your reasons.
Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
Calculate the standard Gibbs free energy change, ΔGΘ, in J, of the redox reaction in (ii), using sections 1 and 24 of the data booklet.
EΘ (BrO3− / Br−) = +1.44 V
State and explain the magnetic property of iron(II) and iron(III) ions.
Markscheme
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
OR
[Ar] 4s2 3d10 4p5 ✔
Accept 3d before 4s.
Accept double-headed arrows.
Structure I - follows octet rule:
Structure II - does not follow octet rule:
Accept dots, crosses or lines to represent electron pairs.
«structure I» formal charge on Br = +2
OR
«structure II» formal charge on Br = 0/+1 ✔
structure II is preferred AND it produces formal charge closer to 0 ✔
Ignore any reference to formal charge on oxygen.
Geometry:
trigonal/pyramidal ✔
Reason:
three bonds AND one lone pair
OR
four electron domains ✔
O−Br−O angle:
107° ✔
Accept “charge centres” for “electron domains”.
Accept answers in the range 104–109°.
BrO3− (aq) + 6e− + 6H+ (aq) → Br− (aq) + 3H2O (l)
correct reactants and products ✔
balanced equation ✔
Accept reversible arrows.
BrO3− (aq) + 6Fe2+ (aq) + 6H+ (aq) → Br− (aq) + 3H2O (l) + 6Fe3+ (aq) ✔
EΘreaction = «+1.44 V – 0.77 V =» 0.67 «V» ✔
ΔGΘ = «–nFEΘreaction = – 6 × 96500 C mol–1 × 0.67 V =» –3.9 × 105 «J» ✔
both are paramagnetic ✔
«both» contain unpaired electrons ✔
Accept orbital diagrams for both ions showing unpaired electrons.
Examiners report
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
Magnesium ions produce no emission or absorption lines in the visible region of the electromagnetic spectrum. Suggest why most magnesium compounds tested in a school laboratory show traces of yellow in the flame.
(i) Explain the convergence of lines in a hydrogen emission spectrum.
(ii) State what can be determined from the frequency of the convergence limit.
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
(ii) Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
(iii) Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
Markscheme
contamination with sodium/other «compounds»
i
energy levels are closer together at high energy / high frequency / short wavelength
ii
ionisation energy
i)
Anode (positive electrode):
2Cl– → Cl2 (g) + 2e–
Cathode (negative electrode):
Mg2+ + 2e– → Mg (l)
Penalize missing/incorrect state symbols at Cl2 and Mg once only.
Award [1 max] if equations are at wrong electrodes.
Accept Mg (g).
ii)
reduction
iii)
Anode (positive electrode):
oxygen/O2
OR
hydogen ion/proton/H+ AND oxygen/O2
Cathode (negative electrode):
hydrogen/H2
OR
hydroxide «ion»/OH– AND hydrogen/H2
Award [1 max] if correct products given at wrong electrodes.
Any two of:
«inert» Pt electrode
OR
platinum black conductor
1 mol dm–3 H+ (aq)
H2 (g) at 100 kPa
Accept 1 atm H2 (g).
Accept 1 bar H2 (g)
Accept a labelled diagram.
Ignore temperature if it is specified.
i
Mg(s) + Cu2+ (aq) → Mg2+ (aq) + Cu(s)
ii
«+0.34V – (–2.37V) = +»2.71 «V»
iii
cell potential increases
reaction «in Q4(k)(i)» moves to the right
OR
potential of the copper half-cell increases/becomes more positive
Accept correct answers based on the Nernst equation
Examiners report
Nitric acid is usually produced by the oxidation of ammonia.
A mixture of nitric acid and sulfuric acid can be used to convert benzene to nitrobenzene, C6H5NO2.
Draw arrows in the boxes to represent the electron configuration of a nitrogen atom.
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
Explain the relative lengths of the three bonds between N and O in nitric acid.
State a technique used to determine the length of the bonds between N and O in solid HNO3.
Write an equation for the reaction between the acids to produce the electrophile, NO2+.
Draw the structural formula of the carbocation intermediate produced when this electrophile attacks benzene.
Deduce the number of signals that you would expect in the 1H NMR spectrum of nitrobenzene and the relative areas of these.
Markscheme
Accept all 2p electrons pointing downwards.
Accept half arrows instead of full arrows.
bonds and non-bonding pairs correct ✔
formal charges correct ✔
Accept dots, crosses or lines to represent electron pairs.
Do not accept resonance structures with delocalised bonds/electrons.
Accept + and – sign respectively.
Do not accept a bond between nitrogen and hydrogen.
For an incorrect Lewis structure, allow ECF for non-zero formal charges.
Any three of:
two N-O same length/order ✔
delocalization/resonance ✔
N-OH longer «than N-O»
OR
N-OH bond order 1 AND N-O bond order 1½ ✔
Award [2 max] if bond strength, rather than bond length discussed.
Accept N-O between single and double bond AND N-OH single bond.
X-ray crystallography ✔
HNO3 + 2H2SO4 NO2+ + H3O+ + 2HSO4- ✔
Accept “HNO3 + H2SO4 NO2+ + H2O + HSO4-”.
Accept “HNO3 + H2SO4 H2NO3+ + HSO4-” AND “H2NO3+ NO2+ + H2O”.
Accept single arrows instead of equilibrium signs.
Accept any of the five structures.
Do not accept structures missing the positive charge.
Number of signals: three/3 ✔
Relative areas: 2 : 2 : 1 ✔
Examiners report
Drawing arrows in the boxes to represent the electron configuration of a nitrogen atom was done extremely well.
Drawing the Lewis structure of HNO3 was performed extremely poorly with structures that included H bonded to N, no double bond or a combination of single, double and even a triple bond or incorrect structures with dotted lines to reflect resonance. Many did not calculate non-zero formal charges.
Poorly done; some explained relative bond strengths between N and O in HNO3, not relative lengths; others included generic answers such as triple bond is shortest, double bond is longer, single longest.
A majority could not state the technique to determine length of bonds; answers included NMR, IR, and such instead of X-ray crystallography.
Many had difficulties writing the balanced equation(s) for the formation of the nitronium ion.
Again, many had difficulty drawing the structural formula of the carbocation intermediate produced in the reaction.
Deducing the number of signals in the 1H NMR spectrum of nitrobenzene, which depend on the number of different hydrogen environments, was done poorly. Also, instead of relative areas, the common answer included chemical shift (ppm) values.
This question is about iron.
Deduce the full electron configuration of Fe2+.
Explain why, when ligands bond to the iron ion causing the d-orbitals to split, the complex is coloured.
State the nuclear symbol notation, , for iron-54.
Mass spectrometry analysis of a sample of iron gave the following results:
Calculate the relative atomic mass, Ar, of this sample of iron to two decimal places.
An iron nail and a copper nail are inserted into a lemon.
Explain why a potential is detected when the nails are connected through a voltmeter.
Calculate the standard electrode potential, in V, when the Fe2+ (aq) | Fe (s) and Cu2+ (aq) | Cu (s) standard half-cells are connected at 298 K. Use section 24 of the data booklet.
Calculate ΔGθ, in kJ, for the spontaneous reaction in (f)(i), using sections 1 and 2 of the data booklet.
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
Markscheme
1s2 2s2 2p6 3s2 3p6 3d6 [✔]
«frequency/wavelength of visible» light absorbed by electrons moving between d levels/orbitals [✔]
colour due to remaining frequencies
OR
complementary colour transmitted [✔]
[✔]
«Ar =» 54 × 0.0584 + 56 × 0.9168 + 57 × 0.0217 + 58 × 0.0031
OR
«Ar =» 55.9111 [✔]
«Ar =» 55.91 [✔]
Note: Award [2] for correct final answer
Do not accept data booklet value (55.85).
lemon juice is the electrolyte
OR
lemon juice allows flow of ions
OR
each nail/metal forms a half-cell with the lemon juice [✔]
Any one of:
iron is higher than copper in the activity series
OR
each half-cell/metal has a different redox/electrode potential [✔]
iron is oxidized
OR
Fe → Fe2+ + 2e−
OR
Fe → Fe3+ + 3e−
OR
iron is anode/negative electrode of cell [✔]
copper is cathode/positive electrode of cell
OR
reduction occurs at the cathode
OR
2H+ + 2e− → H2 [✔]
electrons flow from iron to copper [✔]
«Eθ = +0.34 V −(−0.45 V) = +»0.79 «V» [✔]
«ΔGθ = −nFEθ = −2mol × 96 500 C mol−1 × =» −152 «kJ» [✔]
Note: Accept range 150−153 kJ.
«lnKc = =» 61.38 [✔]
K = 4.5 × 1026 [✔]
Note: Accept answers in range 2.0 × 1026 to 5.5 × 1026.
Do not award M2 if answer not given to two significant figures.
If −140 kJmol−1 used, answer is 3.6 × 1024.
Examiners report
Done fairly well with common mistakes leaving in the 4s2 electrons as part of Fe2+ electron configuration, or writing 4s1 3d5
This was poorly answered and showed a clear misconception and misunderstanding of the concepts. Most of the candidates failed to explain why the complex is coloured and based their answers on the emission of light energy when electrons fall back to ground state and not on light absorption by electrons moving between the split d-orbitals and complementary colour transmitted of certain frequencies.
Many candidates wrote the nuclear notation for iron as Z over A.
This question on average atomic mass was the best answered question on the exam. A few candidates did not write the answer to two decimal places as per instructions.
Very few candidates scored M1 regarding the lemon juice role as electrolyte. Some earned M2 but a lot of answers were too vague, such as ‘electrons move through the circuit’, etc.
Only 50 % of candidates earned this relatively easy mark on calculate EMF from 2 half-cell electrode potentials.
Average performance; typical errors were using the incorrect value for n, the number of electrons, or not using consistent units and making a factor of 1000 error in the final answer.
This question was left blank by quite a few candidates. Common errors included not using correct units, or more often, calculation error in converting ln Kc into Kc value.
Properties of elements and their compounds can be related to the position of the elements in the periodic table.
Explain the decrease in atomic radius from Na to Cl.
Explain why the radius of the sodium ion, Na+, is smaller than the radius of the oxide ion, O2−.
Sketch a graph to show the relative values of the successive ionization energies of boron.
Predict, giving your reasons, whether Mn2+ or Fe2+ is likely to have a more exothermic enthalpy of hydration.
Markscheme
nuclear charge/number of protons/Zeff increases «causing a stronger pull on the outer electrons» ✔
same number of shells/«outer» energy level/shielding ✔
Accept “atomic number” for “number of protons”.
isoelectronic/same electronic configuration/«both» have 2.8 ✔
more protons in Na+ ✔
Sketch showing:
largest increase between third and fourth ionization energies ✔
IE1 < IE2 < IE3 < IE4 < IE5 ✔
Fe2+ AND smaller size/radius
OR
Fe2+ AND higher charge density ✔
stronger interaction with «polar» water molecules ✔
M1 not needed for M2.
Examiners report
Fast moving helium nuclei (4He2+) were fired at a thin piece of gold foil with most passing undeflected but a few deviating largely from their path. The diagram illustrates this historic experiment.
Figure from PPLATO / FLAP (Flexible Learning Approach To Physics), http://www.met.reading.ac.uk/pplato2/h-flap/
phys8_1.html#top 1996 The Open University and The University of Reading.
Suggest what can be concluded about the gold atom from this experiment.
Subsequent experiments showed electrons existing in energy levels occupying various orbital shapes.
Sketch diagrams of 1s, 2s and 2p.
State the electron configuration of copper.
Copper is a transition metal that forms different coloured complexes. A complex [Cu(H2O)6]2+ (aq) changes colour when excess Cl− (aq) is added.
Explain the cause of this colour change, using sections 3 and 15 from the data booklet.
Markscheme
Most 4He2+ passing straight through:
most of the atom is empty space
OR
the space between nuclei is much larger than 4He2+ particles
OR
nucleus/centre is «very» small «compared to the size of the atom» ✔
Very few 4He2+ deviating largely from their path:
nucleus/centre is positive «and repels 4He2+ particles»
OR
nucleus/centre is «more» dense/heavy «than 4He2+ particles and deflects them»
OR
nucleus/centre is «very» small «compared to the size of the atom» ✔
Do not accept the same reason for both M1 and M2.
Accept “most of the atom is an electron cloud” for M1.
Do not accept only “nucleus repels 4He2+ particles” for M2.
1s AND 2s as spheres ✔
one or more 2p orbital(s) as figure(s) of 8 shape(s) of any orientation (px, py pz) ✔
1s22s22p63s23p64s13d10
OR
[Ar] 4s13d10 ✔
Accept configuration with 3d before 4s.
chloride is lower in the spectrochemical series ✔
«ligand cause» decreased/lesser splitting «in d-orbitals compared to H2O» ✔
frequency/energy of light absorbed is decreased
OR
wavelength of light absorbed is increased ✔
Accept ·chloride a weaker ligand than water/produces a smaller energy difference than water for M1.
Award [2 max] for mentioning splitting of orbitals is changed AND frequency/ wavelength/energy of light absorbed
are different/changed without mentioning correct decrease or increase.
Examiners report
When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
Calculate the amount of magnesium, in mol, that was used.
Determine the percentage uncertainty of the mass of product after heating.
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
Calculate coefficients that balance the equation for the following reaction.
Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that would change colour. Use sections 21 and 22 of the data booklet.
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
State the number of subatomic particles in this ion.
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
Suggest, giving a reason, whether magnesium or nitrogen would have the greater sixth ionization energy.
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
Markscheme
2 Mg(s) + O2(g) → 2 MgO(s) ✔
Do not accept equilibrium arrows. Ignore state symbols
aluminium/Al ✔
mass of product ✔
✔
Award [2] for correct final answer
Accept 0.021%
✔
✔
Award «0.2614 mol x 40.31 g mol–1»
Accept alternative methods to arrive at the correct answer.
Accept final answers in the range 90.5-91.5%
[2] for correct final answer.
yes
AND
«each Mg combines with N, so» mass increase would be 14x which is less than expected increase of 16x
OR
3 mol Mg would form 101g of Mg3N2 but would form 3 x MgO = 121 g of MgO
OR
0.2614 mol forms 10.536 g of MgO, but would form 8.796 g of Mg3N2 ✔
Accept Yes AND “the mass of N/N2 that combines with each g/mole of Mg is lower than that of O/O2”
Accept YES AND “molar mass of nitrogen less than of oxygen”.
incomplete reaction
OR
Mg was partially oxidised already
OR
impurity present that evaporated/did not react ✔
Accept “crucible weighed before fully cooled”.
Accept answers relating to a higher atomic mass impurity consuming less O/O2.
Accept “non-stoichiometric compounds formed”.
Do not accept "human error", "wrongly calibrated balance" or other non-chemical reasons.
If answer to (b)(iii) is >100%, accept appropriate reasons, such as product absorbed moisture before being weighed.
«1» Mg3N2 (s) + 6 H2O (l) → 3 Mg(OH)2 (s) + 2 NH3 (aq) ✔
phenol red ✔
Accept bromothymol blue or phenolphthalein.
Mg3N2: -3
AND
NH3: -3 ✔
Do not accept 3 or 3-
Acid–base:
yes AND N3- accepts H+/donates electron pair«s»
OR
yes AND H2O loses H+ «to form OH-»/accepts electron pair«s» ✔
Redox:
no AND no oxidation states change ✔
Accept “yes AND proton transfer takes place”
Accept reference to the oxidation state of specific elements not changing.
Accept “not redox as no electrons gained/lost”.
Award [1 max] for Acid–base: yes AND Redox: no without correct reasons, if no other mark has been awarded
Protons: 7 AND Neutrons: 7 AND Electrons: 10 ✔
isotope«s» ✔
nitride AND smaller nuclear charge/number of protons/atomic number ✔
nitrogen AND electron lost from first «energy» level/s sub-level/s-orbital AND magnesium from p sub-level/p-orbital/second «energy» level
OR
nitrogen AND electron lost from lower level «than magnesium» ✔
Accept “nitrogen AND electron lost closer to the nucleus «than magnesium»”.
Any two of:
subatomic particles «discovered»
OR
particles smaller/with masses less than atoms «discovered»
OR
«existence of» isotopes «same number of protons, different number of neutrons» ✔
charged particles obtained from «neutral» atoms
OR
atoms can gain or lose electrons «and become charged» ✔
atom «discovered» to have structure ✔
fission
OR
atoms can be split ✔
Accept atoms can undergo fusion «to produce heavier atoms»
Accept specific examples of particles.
Award [2] for “atom shown to have a nucleus with electrons around it” as both M1 and M3.
Award [1] for all bonding types correct.
Award [1] for each correct description.
Apply ECF for M2 only once.
Examiners report
Done very well. However, it was disappointing to see the formula of oxygen molecule as O and the oxide as Mg2O and MgO2 at HL level.
Average performance; the question asked to identify a metal; however, answers included S, Si, P and even noble gases besides Be and Na. The only choice of aluminium; however, since its oxide is amphoteric, it could not be the answer in the minds of some.
Very good performance; some calculated the mass of oxygen instead of magnesium for the calculation of the amount, in mol, of magnesium. Others calculated the mass, but not the amount in mol as required.
Mediocre performance; instead of calculating percentage uncertainty, some calculated percentage difference.
Satisfactory performance; however, a good number could not answer the question correctly on determining the percentage yield.
Poorly done. The question asked to evaluate and explain but instead many answers simply agreed with the information provided instead of assessing its strength and limitation.
Mediocre performance; explaining the yield found was often a challenge by not recognizing that incomplete reaction or Mg partially oxidized or impurities present that evaporated or did not react would explain the yield.
Calculating coefficients that balance the given equation was done very well.
Well done; some chose bromocresol green or methyl red as the indicator that would change colour, instead of phenol red, bromothymol blue or phenolphthalein.
Good performance; however, surprising number of candidates could not determine one or both oxidation states correctly or wrote it as 3 or 3−, instead of −3.
Average performance; choosing the given reaction as an acid-base or redox reaction was not done well. Often answers were contradictory and the reasoning incorrect.
Stating the number of subatomic particles in a 14N3- was done very well. However, some answers showed a lack of understanding of how to calculate the number of relevant subatomic particles given formula of an ion with charge and mass number.
Exceptionally well done; A few candidates referred to isomers, rather than isotopes.
There was reference to nitrogen and magnesium, rather than nitride and magnesium ions. Also, instead identifying smaller nuclear charge in nitride ion, some referred to core electrons, Zeff, increased electron-electron repulsion or shielding.
Common error in suggesting nitrogen would have the greater sixth ionization energy was that for nitrogen, electron is lost from first energy level without making reference to magnesium losing it from second energy level.
Good performance; some teachers were concerned about the expected answers. However, generally, students were able to suggest two reasons why matter is divisible.
One teacher commented that not asking to describe bonding in terms of electrostatic attractions as in earlier papers would have been confusing and some did answer in terms of electrostatic forces of attractions involved. However, the question was clear in its expectation that the answer had to be in terms of how the valence electrons produce the three types of bonds and the overall performance was good. Some had difficulty identifying the bond type for Mg, O2 and MgO.
Iron may be extracted from iron (II) sulfide, FeS.
Iron (II) sulfide, FeS, is ionically bonded.
The first step in the extraction of iron from iron (II) sulfide is to roast it in air to form iron (III) oxide and sulfur dioxide.
Outline why metals, like iron, can conduct electricity.
Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
Sketch the first eight successive ionisation energies of sulfur.
Describe the bonding in this type of solid.
State a technique that could be used to determine the crystal structure of the solid compound.
State the full electron configuration of the sulfide ion.
Outline, in terms of their electronic structures, why the ionic radius of the sulfide ion is greater than that of the oxide ion.
Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
Write the equation for this reaction.
Deduce the change in the oxidation state of sulfur.
Suggest why this process might raise environmental concerns.
Explain why the addition of small amounts of carbon to iron makes the metal harder.
Markscheme
mobile/delocalized «sea of» electrons
Any two of:
forms acidic oxides «rather than basic oxides» ✔
forms covalent/bonds compounds «with other non-metals» ✔
forms anions «rather than cations» ✔
behaves as an oxidizing agent «rather than a reducing agent» ✔
Award [1 max] for 2 correct non-chemical properties such as non-conductor, high ionisation energy, high electronegativity, low electron affinity if no marks for chemical properties are awarded.
two regions of small increases AND a large increase between them✔
large increase from 6th to 7th ✔
Accept line/curve showing these trends.
electrostatic attraction ✔
between oppositely charged ions/between Fe2+ and S2− ✔
X-ray crystallography ✔
1s2 2s2 2p6 3s2 3p6 ✔
Do not accept “[Ne] 3s2 3p6”.
«valence» electrons further from nucleus/extra electron shell/ electrons in third/3s/3p level «not second/2s/2p»✔
Accept 2,8 (for O2–) and 2,8,8 (for S2–)
allows them to explain the properties of different compounds/substances
OR
enables them to generalise about substances
OR
enables them to make predictions ✔
Accept other valid answers.
4FeS(s) + 7O2(g) → 2Fe2O3(s) + 4SO2(g) ✔
Accept any correct ratio.
+6
OR
−2 to +4 ✔
Accept “6/VI”.
Accept “−II, 4//IV”.
Do not accept 2- to 4+.
sulfur dioxide/SO2 causes acid rain ✔
Accept sulfur dioxide/SO2/dust causes respiratory problems
Do not accept just “causes respiratory problems” or “causes acid rain”.
disrupts the regular arrangement «of iron atoms/ions»
OR
carbon different size «to iron atoms/ions» ✔
prevents layers/atoms sliding over each other ✔
Examiners report
Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
State the number of protons, neutrons and electrons in each species.
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.
Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
Markscheme
1:2 ✔
Accept 2 Fe3+: 1 Fe2+
Do not accept 2:1 only
mass «spectroscopy»/MS ✔
Award [1 max] for 4 correct values.
specific heat capacity « = » = 0.45 «J g−1 K−1» ✔
Equation:
2Fe3+(aq) + Fe(s) → 3Fe2+(aq) ✔
Cell potential:
«+0.77 V − (−0.45 V) = +»1.22 «V» ✔
Do not accept reverse reaction or equilibrium arrow.
Do not accept negative value for M2.
left electrode/anode labelled zinc/Zn AND right electrode/cathode labelled iron/Fe ✔
electrolyte labelled as «aqueous» zinc salt/Zn2+ ✔
Accept an inert conductor for the anode.
Accept specific zinc salts such as ZnSO4.
« Zn2+» has a full d-shell
OR
does not form « ions with» an incomplete d-shell ✔
Do not accept “Zn is not a transition metal”.
Do not accept zinc atoms for zinc ions.
ligands donate pairs of electrons to metal ions
OR
forms coordinate covalent/dative bond✔
ligands are Lewis bases
AND
metal «ions» are Lewis acids ✔
Examiners report
Oxygen exists as two allotropes, diatomic oxygen, O2, and ozone, O3.
Draw a Lewis (electron dot) structure for ozone.
Discuss the relative length of the two O−O bonds in ozone.
Explain why there are frequencies of UV light that will dissociate O3 but not O2.
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
Markscheme
✔
Accept any combination of lines, dots or crosses to represent electrons.
Do not accept structures that represent 1.5 bonds.
both equal ✔
delocalization/resonance ✔
Accept bond length between 121 and 148 pm/ that of single O−O bond and double O=O bond for M1.
bond in O3 is weaker
OR
O3 bond order 1.5/< 2 ✔
Do not accept bond in O3 is longer for M1.
lower frequency/longer wavelength «UV light» has enough energy to break the O–O bond in O3 «but not that in O2» ✔
Accept “lower frequency/longer wavelength «UV light» has lower energy”.
✔
AND
✔
Do not penalize missing radical.
Accept:for M2:
AND
Examiners report
Analytical chemistry uses instruments to separate, identify, and quantify matter.
Nitric oxide reacts with chlorine.
2NO (g) + Cl2 (g) → 2NOCl (g)
The following experimental data were obtained at 101.3 kPa and 263 K.
Menthol is an organic compound containing carbon, hydrogen and oxygen.
Outline how this spectrum is related to the energy levels in the hydrogen atom.
A sample of magnesium has the following isotopic composition.
Calculate the relative atomic mass of magnesium based on this data, giving your answer to two decimal places.
Complete combustion of 0.1595 g of menthol produces 0.4490 g of carbon dioxide and 0.1840 g of water. Determine the empirical formula of the compound showing your working.
0.150 g sample of menthol, when vaporized, had a volume of 0.0337 dm3 at 150 °C and 100.2 kPa. Calculate its molar mass showing your working.
Determine the molecular formula of menthol using your answers from parts (d)(i) and (ii).
Deduce the order of reaction with respect to Cl2 and NO.
State the rate expression for the reaction.
Calculate the value of the rate constant at 263 K.
Markscheme
electron transfer/transition between high«er» energy level to low«er» energy level
OR
electron transitions into first energy level causes UV series
OR
transition into second energy level causes visible series
OR
transition into third energy level causes infrared series
Accept any of the points shown on a diagram.
24 x 0.786 + 25 x 0.101 + 26 x 0.113
24.33
Award [2] for correct final answer.
Award [0] for 24.31 with no working (data booklet value).
carbon: « =» 0.01020 «mol» / 0.1225 «g»
OR
hydrogen: « =» 0.02042 «mol» / 0.0206 «g»
oxygen: «0.1595 – (0.1225 + 0.0206)» = 0.0164 «g» / 0.001025 «mol»
empirical formula: C10H20O
Award [3] for correct final answer.
Do not award M3 for a hydrocarbon.
«temperature =» 423 K
OR
M
«M » 156 «g mol–1»
Award [1] for correct answer with no working shown.
Accept “pV = nRT AND n = ” for M1.
C10H20O
[1 Mark]
Cl2: first
NO: second
rate = k [NO]2 [Cl2]
180 / 1.80 x 102 «dm6 mol–2 min–1»
Examiners report
Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.
Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
Explain how the catalyst increases the rate of the reaction.
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•H2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of . The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of .
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
Write the half-equation for the formation of gas bubbles at electrode 1.
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
Deduce the half-equation for the formation of the gas identified in (c)(iii).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
Comment on the spontaneity of the disproportionation reaction at 298 K.
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the disproportionation reaction at 298 K.
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
Deduce why the Cu(I) solution is colourless.
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
Markscheme
[Ar] 3d10
OR
1s2 2s2 2p6 3s2 3p6 3d10 ✔
ΔHθ = ΣΔHθf (products) − ΣΔHθf (reactants) ✔
ΔHθ = 2(−241.8 «kJ mol−1») − 4(−92.3 «kJ mol−1») = −114.4 «kJ» ✔
NOTE: Award [2] for correct final answer.
Ea (cat) to the left of Ea ✔
peak lower AND Ea (cat) smaller ✔
«catalyst provides an» alternative pathway ✔
«with» lower Ea
OR
higher proportion of/more particles with «kinetic» E ≥ Ea(cat) «than Ea» ✔
mass of H2O = «18.360 g – 17.917 g =» 0.443 «g» AND mass of CuCl2 = «17.917 g – 16.221 g =» 1.696 «g» ✔
moles of H2O = «=» 0.0246 «mol»
OR
moles of CuCl2 =«= » 0.0126 «mol» ✔
«water : copper(II) chloride = 1.95 : 1»
« =» 2 ✔
NOTE: Accept « =» 1.95.
NOTE: Award [3] for correct final answer.
Wires:
«delocalized» electrons «flow» ✔
Electrolyte:
«mobile» ions «flow» ✔
2Cl− → Cl2 (g) + 2e−
OR
Cl− → Cl2 (g) + e− ✔
NOTE: Accept e for e−.
«electrode» 3 AND oxygen/O2 ✔
NOTE: Accept chlorine/Cl2.
2H2O (l) → 4H+ (aq) + O2 (g) + 4e– ✔
NOTE: Accept 2Cl– (aq) → Cl2 (g) + 2e–.
Accept 4OH− → 2H2O + O2 + 4e−
enthalpy of solution = lattice enthalpy + enthalpies of hydration «of Cu2+ and Cl−» ✔
«+2824 kJ mol–1 − 2161 kJ mol–1 − 2(359 kJ mol–1) =» −55 «kJ mol–1» ✔
NOTE: Accept enthalpy cycle.
Award [2] for correct final answer.
Eθ = «+0.52 – 0.15 = +» 0.37 «V» ✔
spontaneous AND Eθ positive ✔
ΔGθ = «−nFE = −1 mol × 96 500 C Mol–1 × 0.37 V=» −36 000 J/−36 kJ ✔
NOTE: Accept “−18 kJ mol–1 «per mole of Cu+»”.
Do not accept values of n other than 1.
Apply SF in this question.
Accept J/kJ or J mol−1/kJ mol−1 for units.
2 mol (aq) → 1 mol (aq) AND decreases ✔
NOTE: Accept “solid formed from aqueous solution AND decreases”.
Do not accept 2 mol → 1 mol without (aq).
ΔGθ < 0 AND ΔSθ < 0 AND ΔHθ < 0
OR
ΔGθ + TΔSθ < 0 AND ΔHθ < 0 ✔
TΔS more negative «reducing spontaneity» AND stability increases ✔
NOTE: Accept calculation showing non-spontaneity at 433 K.
«ligands cause» d-orbitals «to» split ✔
light absorbed as electrons transit to higher energy level «in d–d transitions»
OR
light absorbed as electrons promoted ✔
energy gap corresponds to «orange» light in visible region of spectrum ✔
colour observed is complementary ✔
full «3»d sub-level/orbitals
OR
no d–d transition possible «and therefore no colour» ✔
octahedral AND 90° «180° for axial» ✔
NOTE: Accept square-based bi-pyramid.
Any two of:
ligand/chloride ion Lewis base AND donates e-pair ✔
not Brønsted–Lowry base AND does not accept proton/H+ ✔
Lewis definition extends/broader than Brønsted–Lowry definition ✔
Examiners report
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:
Outline why ozone in the stratosphere is important.
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
State one analytical technique that could be used to determine the ratio of 14N : 15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
State, giving a reason, the shape of the dinitrogen monoxide molecule.
Deduce the hybridization of the central nitrogen atom in the molecule.
Markscheme
absorbs UV/ultraviolet light «of longer wavelength than absorbed by O2» [✔]
NO (g) + O3 (g) → NO2 (g) + O2 (g) [✔]
NO2 (g) + O3 (g) → NO (g) + 2O2 (g) [✔]
Note: Ignore radical signs.
Accept equilibrium arrows.
Award [1 max] for NO2 (g) + O (g) → NO (g) + O2 (g).
mass spectrometry/MS [✔]
« =» 14.02 [✔]
«Mr = (14.02 × 2) + 16.00 =» 44.04 [✔]
Any two:
same AND have same nuclear charge /number of protons/Zeff [✔]
same AND neutrons do not affect attraction/ionization energy/Zeff
OR
same AND neutrons have no charge [✔]
same AND same attraction for «outer» electrons [✔]
same AND have same electronic configuration/shielding [✔]
Note: Accept “almost the same”.
“Same” only needs to be stated once.
Nitrogen and carbon:
N has greater nuclear charge/«one» more proton «and electrons both lost from singly filled p-orbitals» [✔]
Nitrogen and oxygen:
O has a doubly filled «p-»orbital
OR
N has only singly occupied «p-»orbitals [✔]
Note: Accept “greater e– - e- repulsion in O” or “lower e– - e- repulsion in N”.
Accept box annotation of electrons for M2.
delocalization
OR
delocalized π-electrons [✔]
Note: Accept “resonance”.
linear AND 2 electron domains
OR
linear AND 2 regions of electron density [✔]
Note: Accept “two bonds AND no lone pairs” for reason.
sp [✔]
Examiners report
Candidates sometimes failed to identify how ozone works in chemical terms, referring to protects/deflects, i.e., the consequence rather than the mechanism.
Many candidates recalled the first equation for NO catalyzed decomposition of ozone only. Some considered other radical species.
All candidates, with very few exceptions, answered this correctly.
Most candidates were able to calculate the accurate mass of N2O, though quite a few candidates just calculated the mass of N and didn’t apply it to N2O, losing an accessible mark.
Many students realized that neutrons had no charge and could not affect IE significantly, but many others struggled a lot with this question since they considered that 15N would have a higher IE because they considered the greater mass of the nucleus would result in an increase of attraction of the electrons.
Mixed responses here; the explanation of higher IE for N with respect to C was less well explained, though it should have been the easiest. It was good to see that most candidates could explain the difference in IE of N and O, either mentioning paired/unpaired electrons or drawing box diagrams.
Most candidates identified resonance for this given Lewis representation.
Though quite a number of candidates suggested a linear shape correctly, they often failed to give a complete correct explanation, just mentioning the absence of lone pairs but not two bonds, instead of referring to electron domains.
Hybridisation of the N atom was correct in most cases.
Iron(II) disulfide, FeS2, has been mistaken for gold.
State the full electronic configuration of Fe2+.
Explain why there is a large increase from the 8th to the 9th ionization energy of iron.
Calculate the oxidation state of sulfur in iron(II) disulfide, FeS2.
Describe the bonding in iron, Fe (s).
Markscheme
1s2 2s2 2p6 3s2 3p6 3d6 ✔
Any two of:
IE9: electron in lower energy level
OR
IE9: more stable/full electron level ✔
IE9: electron closer to nucleus
OR
IE9: electron more tightly held by nucleus ✔
IE9: less shielding by «complete» inner levels ✔
–1 ✔
Accept “– I”.
electrostatic attraction/hold between «lattice of» positive ions/cations AND delocalized «valence» electrons ✔
Examiners report
Mostly well done which was a pleasant surprise since this is not overly easy, predictably some gave [Ar] 4s2 3d4.
Despite some confusion regarding which sub-level the electrons were being removed from, many candidates were able to make at least one valid point, commonly in terms of lower energy/ full sub level/closer to nucleus.
This was an easy question, yet 30% of the candidates were unable to work it out; some wrote the oxidation state in the conventionally incorrect format, 1- and lost the mark.
Most candidates knew the bonding in Fe is metallic but some did not “describe” it or missed the type of attraction, a minor mistake; others referred to nuclei or protons instead of cations/positive ions. In some cases, candidates referred too ionic bonding, probably still thinking of FeS2 (not reading the question well). Overall, only 30% answered satisfactorily.
The emission spectrum of an element can be used to identify it.
Hydrogen spectral data give the frequency of 3.28 × 1015 s−1 for its convergence limit.
Calculate the ionization energy, in J, for a single atom of hydrogen using sections 1 and 2 of the data booklet.
Calculate the wavelength, in m, for the electron transition corresponding to the frequency in (a)(iii) using section 1 of the data booklet.
Deduce any change in the colour of the electrolyte during electrolysis.
Deduce the gas formed at the anode (positive electrode) when graphite is used in place of copper.
Explain why transition metals exhibit variable oxidation states in contrast to alkali metals.
Markscheme
IE «= ΔE = hν = 6.63 × 10–34 J s × 3.28 × 1015 s–1» = 2.17 × 10–18 «J»
[1 mark]
«» 9.15 × 10–8 «m»
[1 mark]
no change «in colour»
Do not accept “solution around cathode will become paler and solution around the anode will become darker”.
[1 mark]
oxygen/O2
Accept “carbon dioxide/CO2”.
[1 mark]
Transition metals:
«contain» d and s orbitals «which are close in energy»
OR
«successive» ionization energies increase gradually
Alkali metals:
second electron removed from «much» lower energy level
OR
removal of second electron requires large increase in ionization energy
[2 marks]
Examiners report
The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
Explain why the first ionization energy of sulfur is lower than that of phosphorus.
State the condensed electron configurations for Cr and Cr3+.
Describe metallic bonding and how it contributes to electrical conductivity.
Deduce, giving a reason, which complex ion [Cr(CN)6]3− or [Cr(OH)6]3− absorbs higher energy light. Use section 15 of the data booklet.
[Cr(OH)6]3− forms a green solution. Estimate a wavelength of light absorbed by this complex, using section 17 of the data booklet.
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur tetrafluoride, SF4, and sulfur dichloride, SCl2.
Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Markscheme
nuclear charge/number of protons/Z/Zeff increases «causing a stronger pull on the outer electrons» ✓
same number of shells/«outer» energy level/shielding ✓
P has «three» unpaired electrons in 3p sub-level AND S has one full 3p orbital «and two 3p orbitals with unpaired electrons»
OR
P: [Ne]3s23px13py13pz1 AND S: [Ne]3s23px23py13pz1 ✓
Accept orbital diagrams for 3p sub-level for M1. Ignore other orbitals or sub-levels.
repulsion between paired electrons in sulfur «and therefore easier to remove» ✓
Accept “removing electron from S gives more stable half-filled sub-level" for M2.
Cr:
[Ar] 4s13d5 ✓
Cr3+:
[Ar] 3d3 ✓
Accept “[Ar] 3d54s1”.
Accept “[Ar] 3d34s0”.
Award [1 max] for two correct full electron configurations “1s22s22p63s23p64s13d5 AND 1s22s22p63s23p63d3”.
Award [1 max] for 4s13d5 AND 3d3.
electrostatic attraction ✓
between «a lattice of» cations/positive «metal» ions AND «a sea of» delocalized electrons ✓
mobile electrons responsible for conductivity
OR
electrons move when a voltage/potential difference/electric field is applied ✓
Do not accept “nuclei” for “cations/positive ions” in M2.
Accept “mobile/free” for “delocalized” electrons in M2.
Accept “electrons move when connected to a cell/battery/power supply” OR “electrons move when connected in a circuit” for M3.
[Cr(CN)6]3− AND CN−/ligand causes larger splitting «in d-orbitals compared to OH−»
OR
[Cr(CN)6]3− AND CN−/ligand associated with a higher Δ/«crystal field» splitting energy/energy difference «in the spectrochemical series compared to OH− » ✓
Accept “[Cr(CN)6]3− AND «CN−» strong field ligand”.
any value or range between 647 and 700 nm ✓
SF4/SCl2 structure does not have to be 3-D for mark.
Penalize missing lone pairs of electrons on halogens once only.
Accept any combination of dots, lines or crosses for bonds/lone pairs.
Accept “non-linear” for SCl2 molecular geometry.
Award [1] for two correct electron domain geometries, e.g. trigonal bipyramidal for SF4 and tetrahedral for SCl2.
H2O forms hydrogen bonding «while SCl2 does not» ✓
SCl2 «much» stronger London/dispersion/«instantaneous» induced dipole-induced dipole forces ✓
Alternative 1:
H2O less volatile AND hydrogen bonding stronger «than dipole–dipole and dispersion forces» ✓
Alternative 2:
SCl2 less volatile AND effect of dispersion forces «could be» greater than hydrogen bonding ✓
Ignore reference to Van der Waals.
Accept “SCl2 has «much» larger molar mass/electron density” for M2.
Examiners report
Rhenium, Re, was the last element with a stable isotope to be isolated.
Before its isolation, scientists predicted the existence of rhenium and some of its properties.
One chloride of rhenium has the empirical formula ReCl3.
Rhenium forms salts containing the perrhenate(VII) ion, ReO4−.
The stable isotope of rhenium contains 110 neutrons.
State the nuclear symbol notation for this isotope.
Suggest the basis of these predictions.
A scientist wants to investigate the catalytic properties of a thin layer of rhenium metal on a graphite surface.
Describe an electrochemical process to produce a layer of rhenium on graphite.
Predict two other chemical properties you would expect rhenium to have, given its position in the periodic table.
Describe how the relative reactivity of rhenium, compared to silver, zinc, and copper, can be established using pieces of rhenium and solutions of these metal sulfates.
State the name of this compound, applying IUPAC rules.
Calculate the percentage, by mass, of rhenium in ReCl3.
Suggest why the existence of salts containing an ion with this formula could be predicted. Refer to section 6 of the data booklet.
Deduce the coefficients required to complete the half-equation.
ReO4− (aq) + ____H+ (aq) + ____e− ⇌ [Re(OH)2]2+ (aq) + ____H2O (l) Eθ = +0.36 V
Predict, giving a reason, whether the reduction of ReO4− to [Re(OH)2]2+ would oxidize Fe2+ to Fe3+ in aqueous solution. Use section 24 of the data booklet.
Markscheme
[✔]
gap in the periodic table
OR
element with atomic number «75» unknown
OR
break/irregularity in periodic trends [✔]
«periodic table shows» regular/periodic trends «in properties» [✔]
electrolyze «a solution of /molten» rhenium salt/Ren+ [✔]
graphite as cathode/negative electrode
OR
rhenium forms at cathode/negative electrode [✔]
Note: Accept “using rhenium anode” for M1.
Any two of:
variable oxidation states [✔]
forms complex ions/compounds [✔]
coloured compounds/ions [✔]
«para»magnetic compounds/ions [✔]
Note: Accept other valid responses related to its chemical metallic properties.
Do not accept “catalytic properties”.
place «pieces of» Re into each solution [✔]
if Re reacts/is coated with metal, that metal is less reactive «than Re» [✔]
Note: Accept other valid observations such as “colour of solution fades” or “solid/metal appears” for “reacts”.
rhenium(III) chloride
OR
rhenium trichloride [✔]
«Mr ReCl3 = 186.21 + (3 × 35.45) =» 292.56 [✔]
«100 × =» 63.648 «%» [✔]
same group as Mn «which forms MnO4-»
OR
in group 7/has 7 valence electrons, so its «highest» oxidation state is +7 [✔]
ReO4− (aq) + 6H+ (aq) + 3e− ⇌ [Re(OH)2]2+ (aq) + 2H2O (l) [✔]
no AND ReO4− is a weaker oxidizing agent than Fe3+
OR
no AND Fe3+ is a stronger oxidizing agent than ReO4−
OR
no AND Fe2+ is a weaker reducing agent than [Re(OH)2]2+
OR
no AND [Re(OH)2]2+ is a stronger reducing agent than Fe2+
OR
no AND cell emf would be negative/–0.41 V [✔]
Examiners report
It was expected that this question would be answered correctly by all HL candidates. However, many confused the A-Z positions or calculated very unusual numbers for A, sometimes even with decimals.
This is a NOS question which required some reflection on the full meaning of the periodic table and the wealth of information contained in it. But very few candidates understood that they were being asked to explain periodicity and the concept behind the periodic table, which they actually apply all the time. Some were able to explain the “gap” idea and other based predictions on properties of nearby elements instead of thinking of periodic trends. A fair number of students listed properties of transition metals in general.
Generally well done; most described the cell identifying the two electrodes correctly and a few did mention the need for Re salt/ion electrolyte.
Generally well answered though some students suggested physical properties rather than chemical ones.
Many candidates chose to set up voltaic cells and in other cases failed to explain the actual experimental set up of Re being placed in solutions of other metal salts or the reaction they could expect to see.
Almost all candidates were able to name the compound according to IUPAC.
Most candidates were able to answer this stoichiometric question correctly.
This should have been a relatively easy question but many candidates sometimes failed to see the connection with Mn or the amount of electrons in its outer shell.
Surprisingly, a great number of students were unable to balance this simple half-equation that was given to them to avoid difficulties in recall of reactants/products.
Many students understood that the oxidation of Fe2+ was not viable but were unable to explain why in terms of oxidizing and reducing power; many students simply gave numerical values for EΘ often failing to realise that the oxidation of Fe2+ would have the inverse sign to the reduction reaction.